Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Medical Image Segmentation for Brain Tumor Detection Using U-NET
Authors: Jarpula Sivakumar, Akshay Tonde, Vuyyuru Namitha, S Sangeetha
DOI Link: https://doi.org/10.22214/ijraset.2024.59327
Certificate: View Certificate
Abstract
Brain tumors encompass a wide variety of growths that can occur in the cranial cavity. These tumors can be benign or malignant, and early identification is important for suitable treatment. MRI is a non-invasive imaging method that provides detailed anatomical information and is useful in diagnosing and monitoring brain tumors. However, identifying tumors from complex MRI data remains a difficult and challenging task. This project uses image segmentation techniques to identify and identify tumors on MRI scans. The technology is works on the principles of deep learning, an intelligence process that enables computers to learn and recognize patterns in large data sets. This learning process helps doctors make informed decisions by distinguishing between healthy brain tissue and tumor growth. The unique design of the U-Net architecture, characterized by a U-shaped pattern with compactness and detail, makes it possible to capture complex patterns and fine details in medical images. Using different data from many brain MRI scans, the U-Net algorithm was trained to depict tumor regions with pixel-level accuracy.
Introduction
I. INTRODUCTION
Precise tumor segmentation in medical photographs has great significance since it provides vital information required for cancer analysis, diagnosis, treatment planning and tracking the course of the illness. Based on their origin, brain tumors—which are among the worst malignancies worldwide—are divided into primary and secondary forms. Of all brain tumors that are malignant, gliomas, the most similar type, are derived from brain glial cells and include around 80% of cases. Gliomas can present as aggressive high-grade gliomas (HGG) or glioblastomas, which require rapid intervention, or as slowly developing low-grade (LGG) tumors with a generally good prognosis. Glioblastoma patients, in spite of the best medical and surgical care available, have a median survival of only around 14 months, and their 5-year survival rate is essentially nonexistent. Therefore, prompt diagnosis is essential to successful treatment. The recommended method for evaluating brain tumors is magnetic resonance imaging (MRI), which offers a variety of 3D MRI modalities including fluid-attenuated inversion recovery (FLAIR), T2-weighted, post-contrast T1-weighted (T1ce), and T1-weighted. These modalities reveal distinct subregions of tumors, with FLAIR and T2 highlighting the entire tumor, including infiltrative edema, while T1 and T1ce images depict the tumor core, excluding peritumoral edema. Integrating information from these scans aids in detecting various tumor subregions. A platform for assessing machine learning models targeted at tumor segmentation is offered by the Multimedia Brain Tumor Segmentation Challenge (BraTS). It provides expert-annotated ground facts and a dataset of 3 Dimensional MRI pictures of gliomas (for both LGG and HGG). Automated segmentation of gliomas from multichannel MRI images facilitates precise, repeatable tumor analysis and expedites surgical planning and diagnosis. While unattended studying algorithms and CNNs have become state-of-the-art techniques due to their scope to automatically learn pertinent features, traditional methods entail feature engineering. However, tumor segmentation remains challenging due to tumor heterogeneity in shape, size, appearance, and the ambiguous boundary between cancerous and healthy tissue, compounded by intensity variability in MRI data. Multiple 3D CNN models are used in this work to segment brain tumors from multimodal MRI data. The probability maps of these models as an ensemble improve forecast stability. With optimal hyperparameters, each network is trained independently using the dataset from the 2019 BraTS challenge. The system obtained dice scores of 0.846, 0.906, and 0.750 for the tumor core, total tumor, and enhancing tumor, respectively.
II. OBJECTIVE OF THE PROJECT
In order to analyze diseases and track their course, brain tumors using multimodal MR images must be automatically segmented. Because gliomas are heterogeneous and malignant, it is critical to use precise and effective segmentation methods to divide tumors into intra-tumoral classes. When it comes to tasks like semantic segmentation, deep learning algorithms have proven to perform better than conventional context based on computer perception techniques. With their widespread application in bio-genetic image segmentation, convolutional neural CNNs have made tremendous progress toward the state-of-the-art accuracy for brain tumor separation. In this work, we provide an ensemble process that combines a U-Net and a 3 Dimensional CNN for segmentation. This simple yet effective combination strategy produces better and more precise forecasts. The BraTS-19 challenge dataset was used for each model's independent training and evaluation, which produced segmentation maps with significantly different segmented tumor sub-regions. The final prediction was then produced by variably ensembleing these diverse results. On the validation set, our suggested ensemble obtained accuracy values of 0.750, 0.906, and 0.846 for the detected tumor, complete tumor, and tumor core, respectively. These findings show good performance when compared to current cutting-edge architectures.
III. LITERATURE SERVEY
A. Clinical image examination using MRI for brain tumor research is gaining increasing attention due to the required to evaluate more data efficiently and objectively
Although the first technology for the brain tumor imaging emerged nearly two decades ago, the modern technique has grown and approached modern medicine. This system aims to supply an overview of brain tumors and imaging, starting with a brief introduction. It then examines state-of-the-art segmentation, registration, and modeling techniques for brain imaging of tumors, focusing on gliomas. Segmentation aims to delineate the tumor, its subdivisions and surrounding tissue, while recording and modeling the problems caused by tumor-induced morphological changes. This review examines various methods, considering those suitable for utilize in clinical imaging protocols. Finally, it evaluates the current situation and predicts future developments and trends, paying particular attention to recent uses in radiological evaluation techniques.
B. There are notable national variations in the worldwide event of malignant brain and other CNS tumors, according to research
The objective of this research is to evaluate age and histology changes while estimating distinct histology trends in various parts of the world. The age-adjusted incidence rates (AAIR) per 100,000 years for brain tumors and other CNS malignancies were determined in this study using data from the International Agency for Research on Cancer (IARC) and the Central Brain Tumor Registry of the United States (CBTRUS).
Has made use of. Five countries with cancer. These tumors add to the global burden of cancer-related morbidity and mortality. Variations in cancer incidence among locations may be due to environmental or genetic variables, which will further our knowledge of the disease. For academics, clinicians, illness support groups, and collaborative research, a deeper grasp of the worldwide frequency of brain tumors is crucial.
???????C. The most extensive registry system in the country is maintained by the Central Brain Tumor Registry of the United States (CBTRUS), which collaborates with the National Cancer Institute (NCI) and the Centers for Disease Control (CDC)
For cancers of the central nervous system, including brain tumors. By presenting the most recent population-based data on brain tumors, both malignant and non-malignant, this publication offers accuracy and updates. Age-adjusted rates of incidence and mortality were found in the US population in 2000. Specifically, meningiomas are the most benign tumors, whereas glioblastoma is the most common brain tumor. Disparities in gender and ethnicity are prevalent, and conditions differ across various groups. The report also sheds light on potential future diagnostics and emphasizes the high death rates linked to brain tumors, underscoring the significance of ongoing research and development efforts to enhance patient outcomes.
???????D. When compared to the 2007 classification, the World Health Organization's 2016 Central Classification of Tumor Nervous System is a major improvement
This classification, which combines genetic and histological factors to identify tumors, ushers in a new era in the diagnosis of CNS cancer. Diffuse gliomas, medulloblastomas, and other embryonal tumors undergo a major reorganization characterized by novel formations determined by histological and molecular characteristics. The use of soft tissue grafts in the treatment of fibrous tumors/hemoperycytomas and the addition of brain invasion as a therapy for atypical meningiomas are two significant modifications. In order to enhance patient outcomes, the classification reform seeks to streamline clinical, experimental, and epidemiological research.
???????E. When temozolomide was added to radiation therapy for newly diagnosed glioblastoma, survival significantly improved with little to no additional harm
The study indicated that the combination group had a higher median survival and two-year survival when comparing radiation therapy alone with radiation therapy plus temozolomide. The advantages of combination therapy for survival outweigh the dangers, notwithstanding the possibility of hematological toxic effects in a small percentage of patients. This study offers promise for better results for individuals with glioblastoma, a dangerous illness, and emphasizes the value of various medications in its treatment.
IV. SYSTEM ANALYSIS
A. Existing System
To simplify the brain tumor process, the authors took advantage of the popularity of profound understanding in classifying clinical images and presented a new method combining 3D CNN and U-Net algorithms. Expanding on this basis, a combination of two deep learning methods (CNN and U-Net) is used to enhance the segmentation procedure even more. Each algorithm was trained separately using BRATS brain tumor data, and then its predictions were combined, or concatenated, to create the final segmentation. More importantly, the resulting results show that Dice has a high score, indicating the success of the shape's cross-section. The main goal is to expand a brain segmentation method that increases efficiency and accuracy compared to existing methods. However, despite the promises of the approach, some shortcomings must be acknowledged:
- Lower Accuracy: Although the plan is promising, lower accuracy compared to expectations is still an issue. Despite the high scores mentioned above, there may be cases where the segmentation fails to capture all tumors or misclassifies healthy tissue as tumor, resulting in incorrect diagnosis, monitoring, and treatment planning.
- Computational Complexity: Integrating multiple deep learning algorithms and using hybrid methods will introduce computational complexity, resulting in longer processing times and the need for additional resources. This may make the strategy less scalable, particularly when working with large data or real-time systems that need to adapt quickly.
- Dependency on Training Data: The performance of segmentation depends on the quality and representativeness of the training data in the BRATS dataset. Insufficient or incorrect information can cause the quality of the model to decrease, which can lead to poor performance with missing data or different tumor types
- Sensitivity to Hyperparameters: Deep learning models such as CNN and U-Net are sensitive to hyperparameters and it can be difficult to tune them for optimal performance. Insufficient hyperparameter changes can lead to unsatisfactory segmentation results and even unstable models that require more testing and computational resources.
- Interpretation: Deep learning-based segmentation often lacks interpretation, making it difficult to understand the reasons behind segmentation decisions. This can be a problem for doctors who rely on transparent and explained medical decisions.
- Limitations: The proposed method may have general problems for many or missing data outside the BRATS dataset. Changes in imaging techniques, tumor characteristics, or patient populations can make it difficult for a system to be adaptable and generalizable to multiple sites.
- Overfitting: There is a risk of overextend, perticularly when trading with little or unbalanced data. Rather than learning general features, the model will identify specific patterns for the training data, resulting in decreased operation on unvisible data or new tumors. Control measures may be needed to reduce this risk.
???????B. Proposed System
We use four different images, FLAIR, T1, T2, and T1CE, and the matching annotated segmented image to carry out this job. These pictures are part of a multi-institutional dataset that was obtained from 19 distinct sources and includes multichannel MRI scans for every patient. Tumor subregion delineation is facilitated by the inclusion of T1, T1 contrast enhanced (T1ce), T2-weighted (T2), and Fluid Redused Inversion Recovery (FLAIR) images in the dataset. Preprocessing techniques, such as skull-stripping methods to eliminate non-brain tissue artifacts, are used to guarantee uniformity throughout the data.
Advantages:
- Greater Accuracy: By offering complementing information from various imaging views, the utilize of numerous modalities and a diverse dataset improves the accuracy of tumor segmentation. Tumor delineation accuracy is increased and ambiguity is decreased using this all-encompassing method.
- Improved Visualization: Combining various imaging modalities makes it possible to see the surrounding tissues and tumor boundaries more clearly, which helps doctors determine the precise location of tumor margins and gauge the degree of tumor infiltration.
- Improved Diagnostic Specificity: By integrating various imaging modalities, the segmentation process can achieve higher diagnostic specificity, enabling the identification of subtle differences in tumor characteristics and facilitating more accurate diagnosis and treatment planning.
- Robustness to Imaging Artifacts: Utilizing multiple images mitigates the effect of imaging artifacts and variations in image quality, enhancing the robustness of the segmentation algorithm and ensuring reliable results across different datasets and imaging protocols.
- Comprehensive Tumor Assessment: The combination of FLAIR, T1, T2, and T1CE images allows for a comprehensive assessment of tumor morphology, heterogeneity, and vascularity, providing clinicians with valuable insights into tumor behavior and aiding in personalized treatment strategies.
- Potential for Early Detection: The integration of multimodal imaging data may enable fast detection of subtle tumor changes or recurrence, facilitating prompt intervention and improving patient outcomes.
- Research and Medical Utility: The availability of a diverse and comprehensive dataset enhances the utility of the segmentation model for both research purposes and clinical applications, fostering advancements in brain tumor analysis and treatment optimization.
V. PROPOSED SYSTEM ARCHITECTURE

???????A. Upload the BRATS dataset
The dataset is uploaded for creating the system for U-nets using CNN. The dataset contains 4 different kinds of images for each brain scan. These images include the flair, t1, t1ce, t2. All the images are in black-and-white format only. These images are preprocessed and reduced for efficient processing. For this project, a total of 138 photos were shot, which is a substantial number given that each image is further divided into numerous pixels.
???????B. Generate CNN & U-net
The next stage is to create the CNN and U-net models. In the U-net model, the initial stage is called downsampling, during which the area of the images is decreased. The goal of downsampling is to preserve the image's context; as a result, the feature maps get smaller, making it easier for us to comprehend the image's overall intricacy and structure. A number of RelU-type activation functions and procedures like pooling (such as Max pooling) are used to accomplish downsampling. Each of these layers continues to apply filters to the images, decreasing their dimensionality and producing feature maps that produce information that is similar but more compressed. The process of upsampling comes after the downsampling. Upsampling is done to make localization easier and more accurate. This is used by networks to produce high-resolution images from compressed feature maps. This is critical for both segmentation and boundary drawing to emphasize the areas that have been divided. A sequence of convolutional layers that raise the spatial dimensionality is used to perform the upsampling. Stated differently, it merely expands the feature maps' dimensions. Additionally, it utilizes the feature maps from the earlier downsampling procedure to guarantee the network of high-resolution images (also known as skip connections) and provides accurate localization.
???????C. Upload MRI Image (for Segmentation)
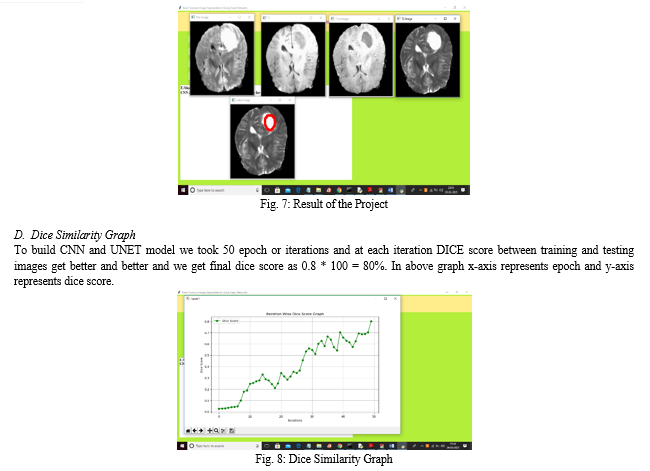
Once the module is generated, we need to upload again 4 different types of images for the same brain scan. These are flair, t1, t1ce, and t2. And the program will output an image with the window title as "label", this shows the actual segmentation that's performed on the image. Once the module is generated, the image is sent for downsampling where it's reduced, then the segmentation takes place during the upsampling part.
???????D. Generate Dice Score
The dice score is a tool for evaluating how well the system or model performs while creating accurate segmentations. The first step in determining the Dice similarity score between two samples is to figure out which pixels in each sample are constant for the intersection, or object of interest. Next, tally every pixel recognized as an item in every pattern. The number of consensus pixels divided by the complete number of pixels in the two samples yields the dice score. This is a straightforward illustration of how well two pieces match; the range is 0 (no match) to 1 (very exact).
VI. MODULES
A. Upload BRATS Dataset
Upload BRATS Dataset is the first module of our project, it is used to upload the BRATS dataset. Click on ‘Upload BRATS Dataset’ button to upload dataset

 ???????
???????
Conclusion
We presented the group of two networks in this study, which are employed together in an ongoing manner to segment biological images. As required by the BraTS 2019 challenge, the group capitally produces a segmentation of brain tumours from the multichannel MRI images that is substantially accurate. This segmentation compares favorably with the predictions provided by other colored state-of-the-art models. To get the style scores, we mix the individual labors from the prototype using a system of variable ensembling. The suggested group provides a clinically useful robotic and objective method for segmenting brain tumors to support complaint planning and case management.
References
[1] \"A check of MRI-predicated clinical image study for brain tumour studies,\" S.Bauer, R.Wiest, L.P.Nolte, M.Reyes, 2013, ( Online). accessible at http// www. [2] \"Global frequence of nasty brain and other CNS tumours by diagnosis, 2003--2007,\" Neuro.Oncol.,vol.19, no.11, pp.1553-1564, 2017, R.Leece, J.Xu, Q.T.Ostrom, Y.Chen, C.Kruchko, and J.S.Barnholtz-Sloan. [3] \"CBTRUS statistical report first brain and CNS excrescences identify in the US in 2005-2009\" (T.A. Dolecek, J.M. Propp, N.E.Stroup, C.Kruchko), Neuro.Oncol., vol. 14, no.suppl_5, pp. v1-- v49, 2012. [4] Acta Neuropathhol.,vol.131, no.6, pp.803-820, 2016. D.N.Louis et al., \"The 2016 World Health Organization type of tumors of the CNS a summary.\" [5] R. Studppetal, \"Adjuvant temozolomide plus attendant radiotherapy for glioblastoma,\" N.Engl.J.Med., vol.352, no.10, pp.987-996,2005. [6] S. Bakasetal, \"Comparing the swish machine knowledge algorithms for brain tumour separation, progression evaluation, and complete survival identification in the BRATS challenge,\"arXiv Prepr.arXiv 1811.02629,2018. [7] \"A generative prototype for brain tumour separation in multichannel images,\" by B.H.Menze, K.Van Leemput,D.-A.Weber,N.Ayache, and P.Golland, in International Conference on Clinical Image Computing and Computer base supported Intervention,2010,pp.151-159. [8] S. Bakas et al., \"Using clever segmentation labels and marconi features to advance the cancer genome atlas glioma glamorous Resonance Imaging collections,\" Sci. data, vol. 4, p. 170117, 2017. [9] Cancer Imaging Archives, vol. 286 (2016); S. Bakasetal., \"Segmentation labels and marconi advances for thepre-operative reviews of the TCGA-LGG collection.\" [10] S. Bakasetal., \"Radiomic features and separation labels for the pre operative reviews of the TCGA-GBM collection.\" The Cancer Imaging Archive, 2017; National Science Data, vol. 4, p. 170117. [11] \"Semantic Segmentation of Brain Tumor from 3 Dimensional Structural MRI Using U-Net Autoencoder,\" M. FARZANA, M. J. HOSSAIN ANY, M. T. REZA, and M. Z. PARVEZ, 2020 International Conference on Machine Learning and Cybernetics (ICMLC), Adelaide, Australia, 2020, pp. 137-142, doi: 10.1109/ICMLC51923.2020.9469580. [12] The article \"Current methods in medical image segmentation\" was published in 2000 by D. L. Pham, C. Xu, and J. L. Prince in the Annual Review of Biomedical Engineering. [13] The article \"Automated medical image segmentation techniques\" was published in the Journal of Medical Physics/Association of Medical Physicists of India on March 3, 2010, and was written by N. Sharma and L. M. Aggarwal. [14] Zhou, Zhou, Tajbakhsh, Siddiquee, M. M. R., and Liang, J., \"Unet++: A nested u-net architecture for medical image segmentation\", Deep learning in medical image analysis and multimodal learning for clinical decision support, pp. 3-11, 2018. [15] The International Journal of Science and Modern Engineering (IJISME) published a paper by P. Sapra, R. Singh, and S. Khurana titled \"Brain tumor detection using neural network\" in 2013. [16] Pattern Recognition Letters, 2017, J. Amin, M. Sharif, M. Yasmin, and S. L. Fernandes, \"A distinctive approach in brain tumor detection and classification using MRI\". [17] \"A new method for brain tumor identification with MRI images,\" S. Zhang and G. Xu, Journal of Biomedical Science and Engineering,vol.9,no.10, pp.44-52,2016. [18] \"Brain Tumour Detect and Separation using CNN Architecture and U-Net Architecture,\" 2023 International Conference on Integrated Intelligence and Communication Systems (ICIICS), Kalaburagi, India, 2023, pp. 1-5, doi: 10.1109/ICIICS59993.2023.10421540, S. R. Totad, Meeradevi, B. J. Sowmya, P. Boppudi, N. Yaswanth, and I. Nivedith. [19] \"Effective MRI based Brain Tumor Detection using Modified U-Net Model,\" M. M. Nayak and S. D. K. A., 2022 IEEE 2nd Mysore Sub Section International Conference (MysuruCon), Mysuru, India, pp. 1-7, doi: 10.1109/MysuruCon55714.2022.9972503. [20] \"A LeakyReLU based Effective Brain MRI Segmentation using U-NET,\" M. V. S. Lakshmi, P. L. Saisreeja, L. Chandana, P. Mounika, and P. U, 2021 5th International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 2021, pp. 1251-1256, doi: 10.1109/ICOEI51242.2021.9453079.
Copyright
Copyright © 2024 Jarpula Sivakumar, Akshay Tonde, Vuyyuru Namitha, S Sangeetha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59327
Publish Date : 2024-03-23
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

